
Scientific Program
ECaPPH aims to transform the ecosystem by leveraging existing and future infrastructure and platforms to amplify the proactive pandemic preparedness chain. By strengthening existing platforms and technologies and promoting the integration of artificial intelligence (AI), ECaPPH-supported projects will amplify the development, production, evaluation and manufacturing of vaccines, including RNA-based, small molecule and cell-based therapies for emerging infections, while optimizing surveillance and the training of a new workforce. The resulting ecosystem must be comprehensive, robust, agile and versatile. Indeed, we do not know the etiological nature of the agent that will be responsible for the next pandemic, nor the most effective countermeasures to combat it. Therefore, the ECaPPH has adopted a proactive chain of pandemic preparedness. The entire Canadian ecosystem will be strengthened by filling the significant gaps in this innovation chain identified during the COVID-19 pandemic. Having a variety of proven and established strategies in our preparedness toolbox will enable ECaPPH partners to be agile in implementing the most rapid and effective response.
TABLE OF CONTENTS
Major Themes
- Small molecules, immunomodulators and cell therapies
- Genomics-based diagnostics and RNA-based therapies for emerging infections
- Vaccine development, production and evaluation
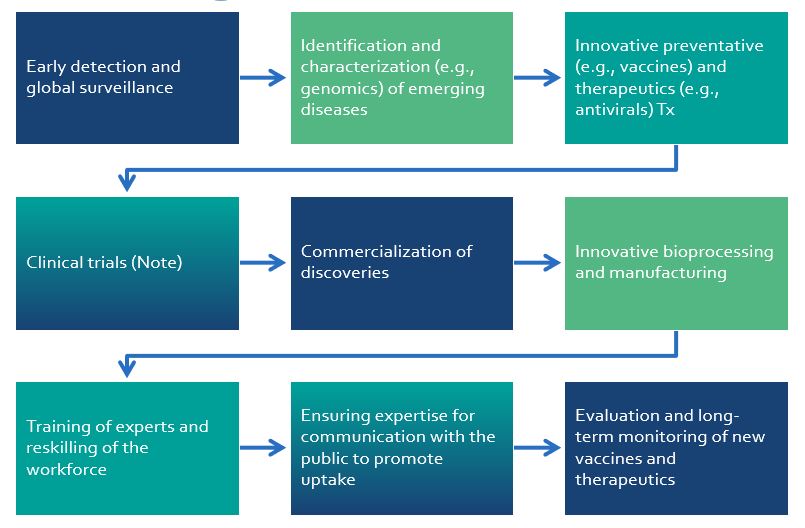
| Establish sustainable linkages with global surveillance groups, and develop strategies for early detection |
| Optimize the use of biobanking data (samples, pathogens, databases) |
| Promote the development, testing and manufacturing of novel diagnostic methods for pathogen detection and host response |
| Accelerate the use of RNA-based approaches and therapies against pandemic pathogens |
| Develop immune-based approaches, such as vaccines, biologics and cell therapies against pandemic pathogens; and RNA-based approaches, virology research, vaccines, therapeutics, biologics, cell therapies, AI, preclinical studies and digital platform for conducting virtual clinical trials emerging pathogens, including identification (Dx), preventive (e.g., vaccines) and therapeutic (e.g., antiviral, anti-inflammatory and supportive therapies) solutions |
| Invest in new strategies to develop and produce novel therapeutics |
| Provide the capacity for state-of-the-art in vitro, in vivo and ex vivo preclinical models of pandemic pathogens |
| Create platforms for rapid clinical trials to respond to an emerging threat |
| Stimulate the bioprocessing and manufacturing of small molecules, nucleic acids, recombinant proteins, viral vectors and human cells to provide a complete armamentarium against pandemic pathogens |
| Instill a tradition of impact by facilitating entrepreneurship and dialogue with decision and policy makers, and the general public |
| Strengthen the workforce capacity and new talents in pandemic preparedness and biomanufacturing |
| Facilitate collaboration with governmental organizations and policy makers, commercialization of discoveries, strategic partnerships, and entrepreneurship |
| Ensure expertise for communication with the public to ensure adoption |
| Support the biomanufacturing ecosystem following current good manufacturing practices |
Ce contenu a été mis à jour le 28 March 2024